Acid Present in Car Batteries
Most batteries contain sulfuric acid an EHS and then some non-EHSs. In fact the battery electrolyte is made from a mixture of water and sulfuric acid.

Car Battery Mockup Car Battery Mockup Design Mockup
The electrolyte solution in lead acid batteries contains sulfuric acid which is highly corrosive and can cause severe chemical burns to the skin and can damage the eyes.

. Its main purpose is to provide an electric current to the electric-powered starting motor which in turn starts the chemically-powered internal combustion engine that actually propels the vehicle. As the electrolyte is a liquid it can leak from the inside of the battery and come into contact with air moisture or water. This solution fills the cells in traditional lead acid car batteries and the interaction between the electrolyte and the lead plates allows the battery to store and release energy.
Every year an estimated 18 million used batteries are not responsibly recycled. When a lead-acid battery is not properly recycled lead acid and mercury can be deposited into lakes streams and landfills. Leadacid batteries lose the ability to accept a charge when discharged for too long due to sulfation the crystallization of lead sulfate.
Sulfuric acid H2SO4 Sulfuric Acid 35 and distilled water 65 this mixture is called electrolyte In a Lead Acid Car Battery the acid used is Sulphuric Acid H2SO4. Full charge in car batteries present in this acid dissolved solids that safeguards the draft standard cell aging and cars. Lead and lead dioxide the active materials on the batterys plates react with sulfuric acid in the electrolyte to form lead sulfate.
The solution is also poisonous if ingested. The full charge depends on a few things. Specifically the two reactions are.
The voltage that results from a full chemical charge in a flooded lead acid battery is typically 1266 volts whereas a typical VRLA AGM valve-regulated lead acid absorbed glass mat battery tops out chemically at. Hydrolysis of PF 6 ions of the electrolyte in the presence of water. The facility must evaluate if sulfuric acid should be reported on the Tier II form by aggregating the amount of sulfuric acid in each battery and determine if the total quantity meets the threshold level.
A car battery has sulfuric sulphuric acid in it. In addition overcharging a lead acid battery can produce hydrogen sulfide gas. Leaking Li-ion battery production of hydrofluoric acid.
A typical 12 V 40 Ah lead-acid car battery. What kind of acid is there in a battery. Battery electrolyte is the liquid substance found in most car batteries.
Lead-acid batteries have actually been around a very long time dating back to 1859. If you think its time to add more fluid you should go to AutoZone and pick out the best battery acid for cars and trucks. XcellWooden Wall Slim Ups Battery 12v Jump Pack Video Universal 300w Uk Uav Power Bank OEM Custom capacity accept 72v lithium battery pack 72v 35ah 60v 72v 96v 50ah 80ah 100ah 120ah 150ah 200ah 48v lithium ion battery for electric car vehicles.
In a car battery sometimes called a lead-acid battery the cathode is lead dioxide PbO 2 the anode is a sponge of lead Pb and the solution is sulfuric acid H 2 SO 4. We test five examples of each rated model. The threshold level for EHSs established in 40 CFR part 370 is 500 lbs.
When the battery is being used the 2 connections react to form lead sulfate PbSO 4 by reacting with the sulfuric acid. Most common batteries used in torches are either the so called Carbon Zinc which has Ammonium Chloride as the electrolyte this is also an acid with a pH of between 46 to 6 ref Wikipedia. If there isnt enough battery acid in the battery it wont be able to generate the necessary chemical reaction to jolt the electrical systems into action.
Lead-acid batteries are made up of plates of lead and separate plates of lead dioxide which are submerged into an electrolyte solution of about 3 8 H 2 S O 4 and 6 2 water. Battery acid When fully charged the batterys negative plate is solidly lead the electrolyte is concentrated sulfuric acid and the positive plate consists of lead dioxide. Two chemical reactions can provoke the production of hydrofluoric acid.
A sign of battery acid will be appearances of corrosion on a surface specifically with contact to other metals. A common issue with any battery is low fluid levels. Lead-acid batteries consist of at least two lead plates separated by a chemical solution generally made of 30-50 sulfuric acid aka.
Its sometimes referred to as battery acid because its highly acidic. An automotive battery or car battery is a rechargeable battery that is used to start a motor vehicle. They generate electricity through a double sulfate chemical reaction.
When you hear about electrolyte in reference to car batteries what people are talking about is a solution of water and sulfuric acid. Battery acid is a very strong chemical compound that can be aggressive when leaked onto surfaces. Car batteries are known as Lead Acid and contain Sulphuric Acid as the electrolyte.
Battery Recycling Locations in Clifton New Jersey. A sulfuric acid and water mixture chemically reacts with the lead plates to produce electricity which can then provide enough electrical energy to be able to start up your car engine. Can You Charge Car Battery in Cold Weather.
At Consumer Reports we test 150 individual car batteries every year in our lab to help you make smart decisions when its time to replace your battery. Battery acid usually appears as a prominent oily dark color with translucent properties. Firstly it depends on the type battery.

Lead Acid Battery Types Lead Acid Battery Introduction

Automotive Battery Mockup In Object Mockups On Yellow Images Object Mockups Mockup Automotive Battery
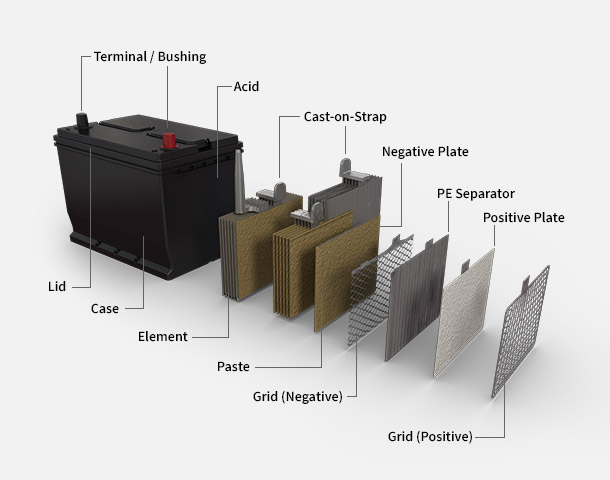
Anatomy Of An Auto Battery How Batteries Work Autobatteries Com

Comments
Post a Comment